Marketing Development
Hawthorne Effect
Transforming Clinical Trials
Client: Hawthorne Effect
Services: Branding, Marketing
Background
The clinical trial industry faces significant challenges, including participant recruitment, retention, and accessibility. Hawthorne Effect sought a brand presence that reflected their groundbreaking approach while fostering trust among researchers, sponsors, and patients.
Approach
To address these challenges, I developed a strategic brand identity aligned with Hawthorne Effect’s mission. We crafted a compelling narrative highlighting their commitment to innovation and patient-centric trials, supported by a modern visual language that conveys trust and credibility.
The marketing strategy boosted brand awareness and engagement, while an intuitive digital experience streamlined access to key information.
Results & Impact
Through a blend of strategic branding, innovative design, and targeted marketing efforts, we helped Hawthorne Effect amplify their impact and establish a strong foundation for continued growth in the healthcare technology sector.




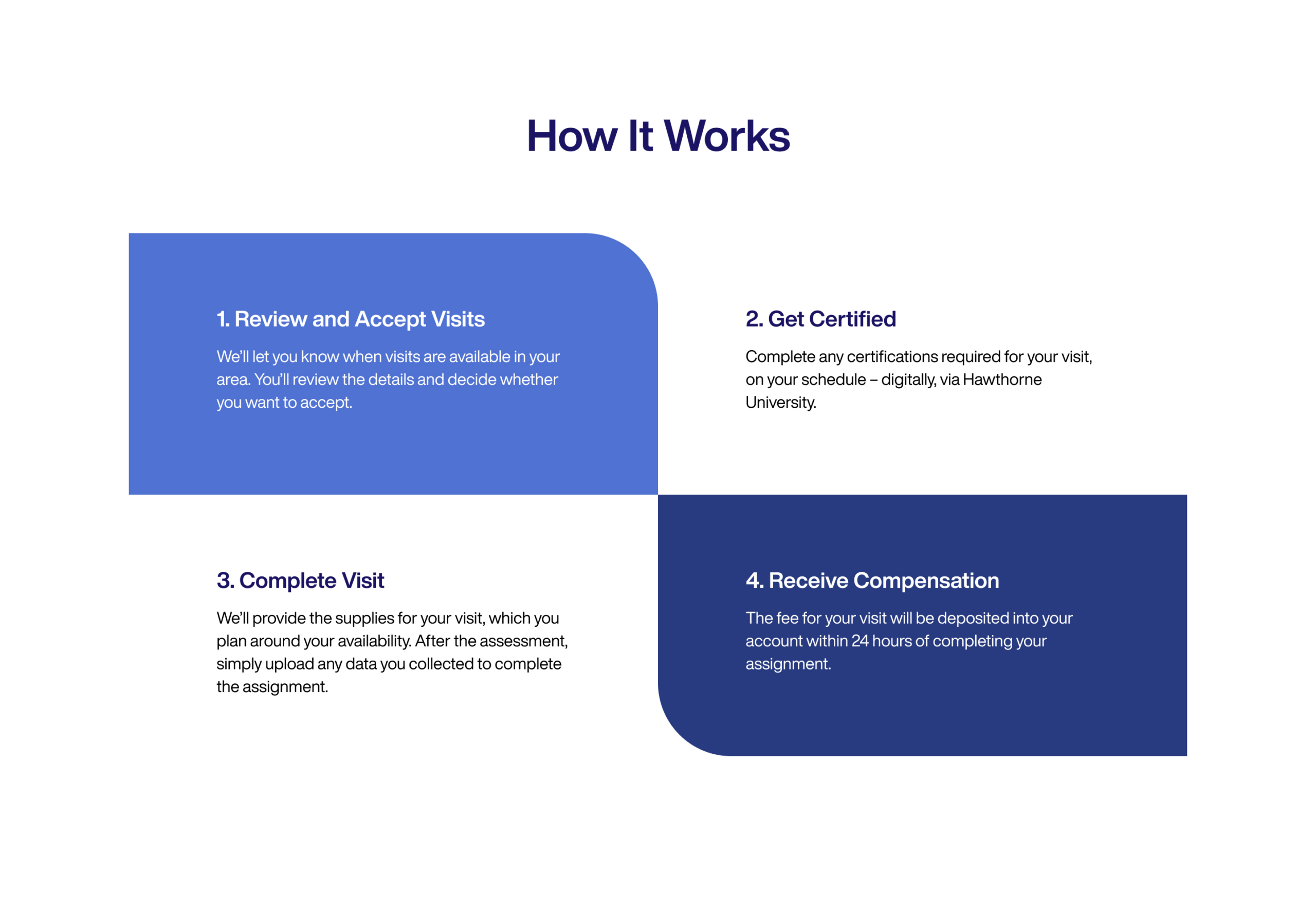
I led the redesign of the Hawthorne Effect website to better communicate the brand and serve as a central reference point for the broader community. Beyond the public-facing experience, I also worked closely with the product and engineering teams to integrate a secure login system that allowed team members to manage their accounts directly from the site. While this functionality was technically part of a separate product, the unified experience made it easier for users by consolidating everything into a single, cohesive platform.

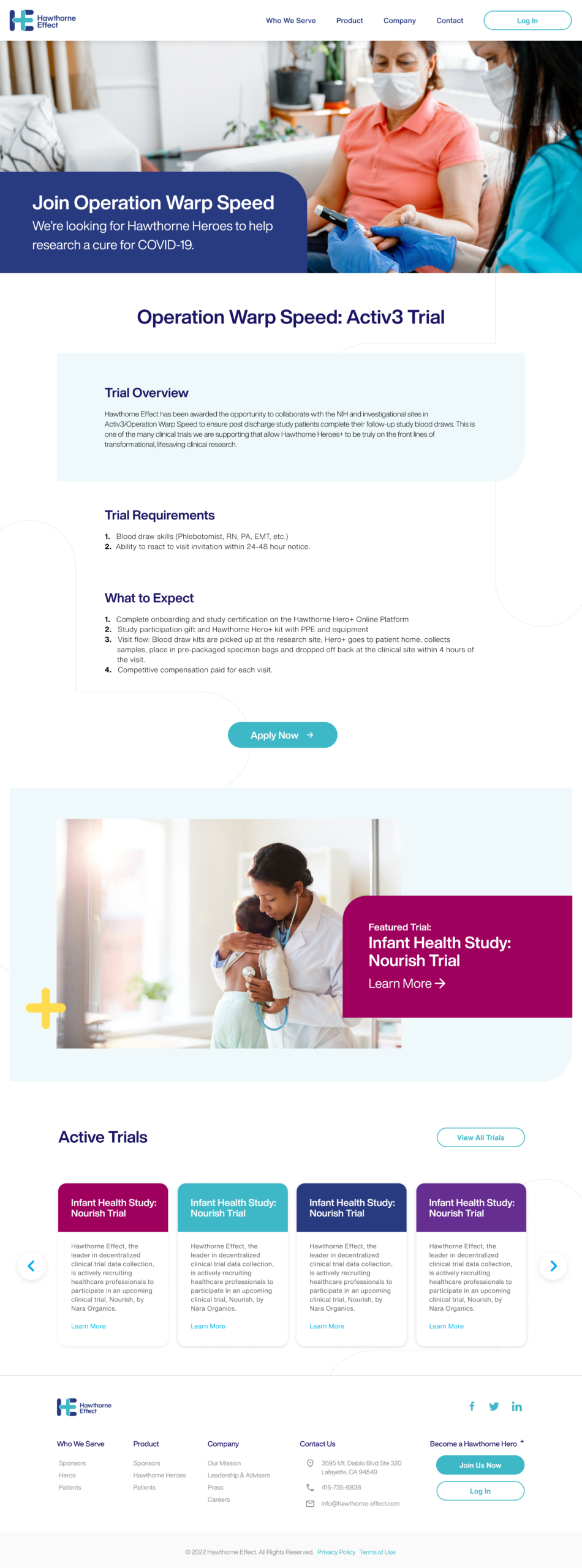
The new design clearly outlined the steps to becoming a Hawthorne Hero—a title introduced by the CEO and Founder before the pandemic, recognizing individuals who participate in advancing clinical research.

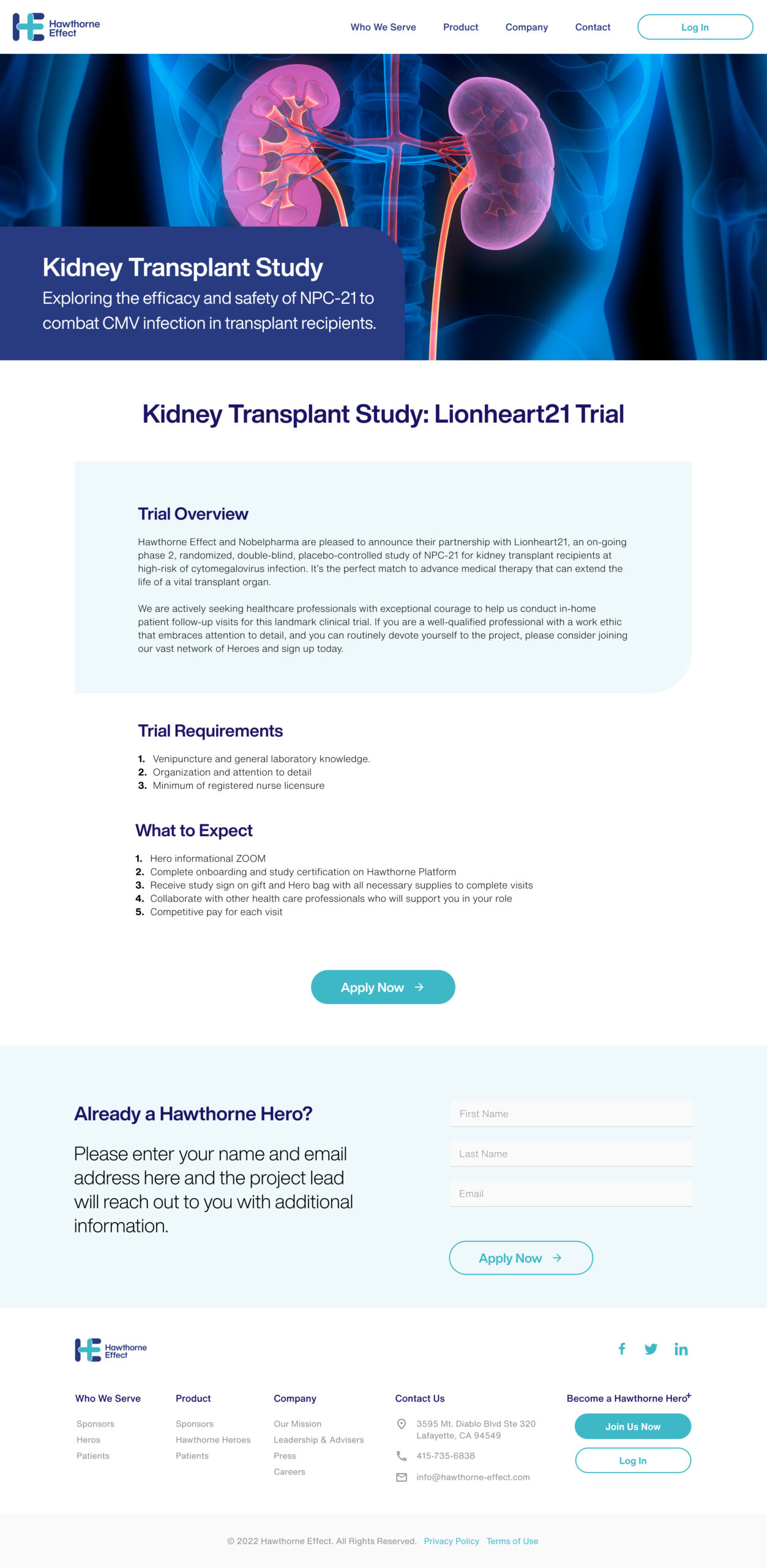
I partnered with Hawthorne Effect to support Operation Warp Speed, the U.S. government initiative to accelerate COVID-19 vaccine development and distribution. My role involved designing and refining digital experiences that streamlined remote clinical trials and decentralized patient monitoring.
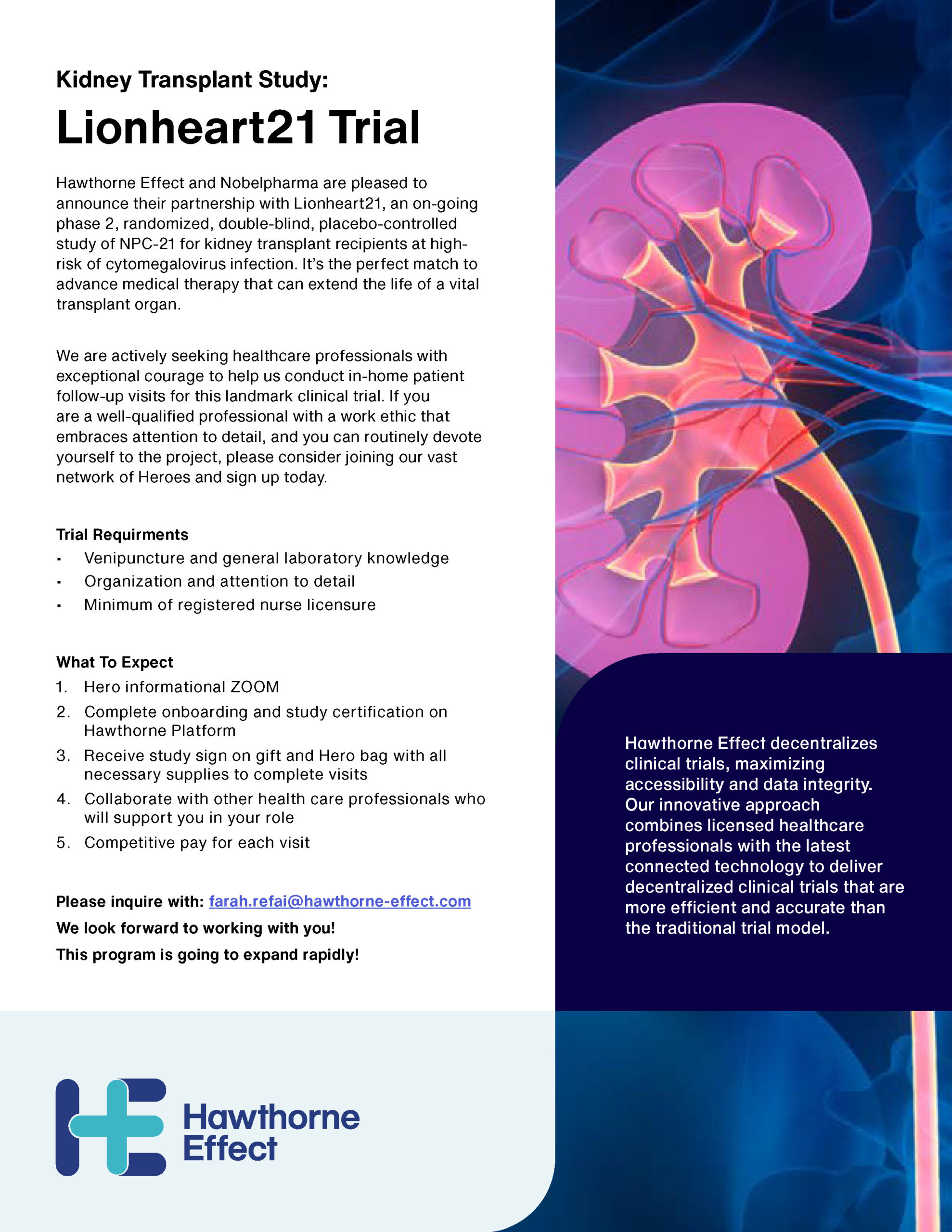
In addition to optimizing the digital platform, we developed a comprehensive collateral system to support clinical trial operations across diverse study sites. This included branded onboarding materials, instructional guides, consent forms, and printable resources designed to ensure consistency, clarity, and accessibility for both patients and healthcare professionals. These materials played a key role in reinforcing trust, streamlining trial execution, and ensuring regulatory alignment across remote and decentralized environments.

We created a series of social media posts that highlighted their critical role in Operation Warp Speed and their broader mission to revolutionize clinical trials.
The website also served as the digital backbone for decentralized trials. These pages enabled efficient patient onboarding, data collection, and remote monitoring. My approach focused on scalable architecture to accommodate multiple trials simultaneously.